
Synthesis of Nano-structured Ferroelectric Tetragonal BaTiO3
Abstract
The Barium Titanane (BaTiO3) nano-particles were synthesized by aqueous co-precipitation technique. The X-ray Diffraction (XRD) results showed that the BaTiO3 powder has particle size of 50 nm and tetragonal (P4mm) perovskite structure at room temperature. The sample has also been characterized by Transmission Electron microscopy (TEM), TGA-DSC, FTIR and P-E hysteresis loop measurements.
Keywords: X-ray diffraction, Transmission Electron Microscopy and ferroelectric hysteresis (PE) loop measurement.
The nano ferroelectric ceramics are important electronic materials due to their wide range of industrial and commercial applications, such as high-dielectric constant capacitors, piezoelectric sonar or ultrasonic transducers, pyroelectric security sensors, medical diagnostic transducers and electro-optical light valves etc. The Barium titanate (BaTiO3) is a member of the perovskite family [1].
Barium titanate (BaTiO3) is the first discovered ferroelectric perovskite. Its ferroelectric properties are connected with a series of three structural phase transitions. The most investigated phase transition is from tetragonal ferroelectric to cubic para-electric structure which occurs at Curie temperature TC =120 oC. The ferroelectricity is fundamentally associated with domain structure and domain motion. The domain structure is formed during the cubic (paraelectric phase) to tetragonal phase (ferroelectric phase) transformation at the Curie temperature. The values of the dielectric constant depend on the synthesis route, temperature, frequency and dopants. The synthesis method depends on the desired characteristics for the end application. The grain size (microstructure) is a very important parameter which has an influence on the The aim of the present work is to synthesize nano-structured BaTiO3 with stabilized tetragonal crystal structure at room temperature, which is an essential requirement for the ferroelectricity and it is confirmed by FTIR spectra, X-ray diffraction and P-E loop measurements.
[2.0] Experimental :
The nano BaTiO3 was prepared by Co-precipitation technique [2] from aqueous solution, in which the reactants were mixed in one molar stoichiometric quantity. The starting raw materials were BaCl2·2H2O and TiCl4. The precipitants NH4HCO3 and NH3·H2O were added with constant stirring to this solution (pH= 11). After the filtrations and the precipitate was washed several times and dried in an oven at 100 °C for an hour. The sample was characterized by XRD, TEM, TGA-DSC, FTIR and P-E loop measurements.
[3.0] Result and Discussion :
The XRD pattern (fig. 1) confirmed the Monophasic pure crystal structure of nano BaTiO3 powder as tetragonal and space group P4mm (JCPDS card No. 31-0174). The lattice parameters are a = b = 4.034 Ǻ and c = 4.064 Ǻ. The earlier workers have observed coexistence of tetragonal (66.3%) and orthorhombic (33.7 %) phases in nano-structured BaTiO3 [3]. Barium Titanate- BaTiO3 has a primitive cubic lattice and a “basis” with atoms, Ba, Ti and O having fractional co-ordinates as follows:
Ba :(0,0,0 ) , Ti : (½,½,½),
and
O : (½,½,0,), (½,0,½), (0,½,½)
The XRD intensity depends upon the structure factor ,
![]()
For example, by using the above coordinates for Ba, Ti and O, the X-ray structure factor for the (00l) Bragg reflection can be written as:
![]()
Where, ![]() ,
,
![]() and
and ![]() are the atomic scattering factors of barium, titanium and oxygen, respectively. Since the XRD intensity is proportional to square of the structure factor, one can prove that the (002) intensity should be significantly higher than that of (001). This is seen in the XRD patterns of ceramic as well as in the pattern of wet-chemically prepared nano-sized sample.
are the atomic scattering factors of barium, titanium and oxygen, respectively. Since the XRD intensity is proportional to square of the structure factor, one can prove that the (002) intensity should be significantly higher than that of (001). This is seen in the XRD patterns of ceramic as well as in the pattern of wet-chemically prepared nano-sized sample.
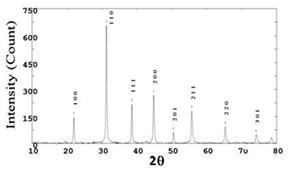
Fig. 1: XRD of nano-sized BaTiO3 powder.
It is known that, simultaneous TGA-DTA measures both heat flow and weight changes (TGA) in a material as a function of temperature in a controlled atmosphere. The complementary information obtained allows differentiation between endothermic and exothermic events with no associated weight loss (e.g. melting and crystallization) and those that involve a weight loss (e.g. decomposition). Fig.2 shows TGA-DSC curves of wet-chemically prepared sample of BaTiO3.The endothermic peaks at 950C and 5760C observed are appears to be associated with weight loss. The TGA thermogram reveals two major decrease in specimen weight in the range 500C to 1500C, and 5000C to 6500C. Both the weight losses corresponds to DTA endotherms. No exothermic peak is observed.
he initial weight loss within the temperature range 500C to 1500C is due to removal of chloride impurities which is generally accepted mechanism of decomposition for BaTiO3 specimen prepared by co-precipitation or hydrothermal route using chlorides of Barium and titanium[5].

Fig.2:TGA-DSC curves of nano-structured BaTiO3
The TEM image (fig. 4) shows an assembly of quasi-spherical BaTiO3 nano-particles which appears to be partially agglomerated owing to the absence of surface stabilizing agent or ligands. The particle found through TEM is 50 nm which is in good agreement with the particle size found from the XRD using the Scherer’s formula[4]. The dielectric constant (ε) was determined through Agilent made Precision LCR meter at 300 K and frequency of 1 kHz was 2300. The frequency dependence exhibited the universal response. The TGA-DSC showed initial weight loss within the temperature range 500C to 1500C due to removal of water content and hydroxyl ions and the prominent weight loss in the temperature range 5000C to 6500C corresponds to removal of chloride impurities which is generally accepted mechanism of decomposition for BaTiO3 specimen prepared by co-precipitation or hydrothermal route using chlorides of Barium and titanium.
The FTIR spectrum (fig. 5) shows a broad absorption in a wide range from 2400 to 3800 cm_1 indicated the presence of H2O and OH- in the BaTiO3 nanoparticles. A large broad band is observed at around 3400 cm_1 and 2923 cm-1 for the surface-adsorbed hydroxyl group. The absorption bands observed near 1600 cm-1 are due to organic impurities. The sharp bands observed near 1400 cm-1 and 1100 cm-1 are due to C-O bonded to Ti ions. The absorption band at 800 cm-1 is due to metal-oxygen ion stretching vibrations. The vertical Ti-O chain with two half-Oxygen atoms and four Oxygen half atoms at right angle to the axis gives rise to two normal IR active vibrations. The IR bands at 588.5 cm-1 and 433.5 cm-1 are observed corresponding to pure tetragonal phase. The IR-absorption band, well below our experimental range, which should be at around 250 cm-1 is due to Ba- (TiO3) vibration. Fig.3 shows two IR active normal modes of vibrations in TiO6 octahedra. The related force constants are calculated for these two frequencies, 588.5 cm -1 and 433.5 cm -1 are k1 = 0.818 x 105 dyne/cm and k2 = 689 x 105 dyne/cm, respectively. The IR spectroscopy confirms the existence of pure tetragonal phase stabilized at room temperature.
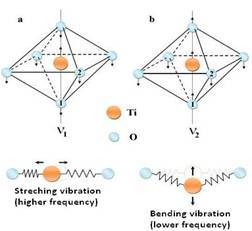
Fig.3 Stretching(a) and bending(b) vibrations in TiO6 octahedron
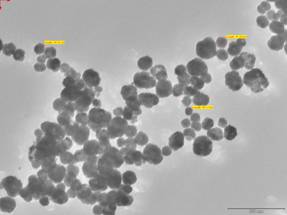
Fig 4. TEM of nano BaTiO3 powder
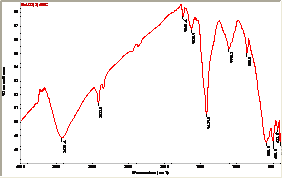
Fig 5. FTIR of nano BaTiO3 powder
The ferroelectric properties deduced from P-E loop (fig. 6) are as follows: Ps = 7.6 μC/cm2, Ec = 4.9 kV/cm and Pr = 2.7 μC/cm2.
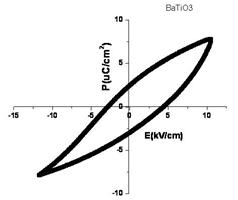
Fig. 6. Ferroelectric loop for the nano BaTiO3 Sample.
4.0 Conclusion
The nano BaTiO3 powder (50 nm) with stabilized tetragonal crystal structure was successfully prepared by co-precipitation technique as confirmed by XRD, FTIR and ferroelectric hysteresis parameters.
REFERENCES :
***************************************************
Ashish R. Tanna
Kushal Vala
Jagdish D. Baraliya
Hiren H. Joshi



Home |
Archive |
Advisory Committee |
Contact us