
Interfacial Energy and Inductance Time Measurement Studies on Thiourea Single Crystal
Abstract
There is practically no one front line technology which does not have requirement of single crystals. Single crystals are the pillar of modern technology. The initiations of good seed organic single crystals are very much difficult. The controlling parameters of growth of single crystal from solution are the constitutes of the material to be crystallized are dissolved in suitable solvent and crystallization starts as the solution becomes critically supersaturated. The super saturation may be promoted by evaporation of the solvent by cooling the solution or by a transport process in which the solute is made to flow from hotter to a cooler region. The precipitation of solid material from liquid solution is a classical method for growing crystal. The present authors have adopted the relatively simple and inexpensive slow evaporation aqueous solution technique for the growth of technological important single crystal of thiourea (NH2 CS NH2) crystal. To understand this growth kinetics of the crystal is made to estimate the interfacial energy and inductance time for such crystal. The results are reported in detail in present communication.
Key Words: - Organic crystal, Thiourea , evaporation technique interfacial energy induction time.
1.0 Introduction
The study and engineering of crystal facet have attracted immense interest of artists and crystal grower community since at least the Bronze Age [1]. In recent trends of technology there has been increased need of new materials for non-linear optical (NLO) applications. The search for new NLO crystals, resulted in the development of new type of material called organic and semi organic materials, which possess sufficiently large nonlinear coefficient, transparency in uv region. Organic materials with High polarizability have great potential to combine with mechanically strong and thermally stable inorganic material to form semiorganic materials, which have parameters competitive with widely used crystals of KDP type [2]. The studies of organic and semiorganic crystals morphology are important for its device application, in which only some particular facet are usable for pharmaceutical industries. Although significant efforts have been made for the last few decades, to predict precisely the growth morphology of crystals, however, it is a still challenging task to this date. The study of growth phenomena, growth kinetics and crystal morphology have been a matter of great research interest for centuries [3]. With the growing importance of nanotechnology, however this field of research is expected to become even more relevant. As the surface to volume ratios diminish in miniaturized devices, surface effects become increasingly important and strongly influence the state of the systems. Many authors had attempted a direct estimation of interfacial tensions from computer simulation[4] . Hartman and Perdok attempted to quantify crystal morphology in terms of the interaction energy between crystallising units [5] . They used the assumption proposed by Born that surface energy is directly related to the chemical bond energies.Thiourea (NH2CSNH2) is one of the organic materials that have high potential for these applications. This material has good chemical flexibility to provide nonlinearity of organic material and strong mechanical property of inorganics to form semiorganic materials. The crystals of Thiourea are hexagonal and tetragonal in shape with the cell parameters are a=7.6610Ǻ, b= 8.5767Ǻ, c=5.492Ǻ. Thiourea crystal is soft and fairly transparent. It is a promising organic crystal that found practical applications in nonlinear optics (NLO) to date, because it is transparent down to 200 nm and has fairly large birefringence, so that phase matching for second harmonic and frequency mixing processes can be achieved well into the UV region [6].So active research work is quite required to understand the fundamental growth and nucleation mechanism of Thiourea material. It is also essential to understand the growth phenomena in order to predict the growth rate and the thickness of the grown crystals. But there are few reports available in the literature on the nucleation kinetics and a few literature reports are available on the growth kinetics of Thiourea single crystals from solutions. In the present investigations the author had tried to evaluate the interfacial tension (σ) between Thiourea crystal and water based solution by measuring the induction period and hence to calculate the critical radius (r*), number of molecules in the critical nucleus (i*) and Gibb's free energy (ΔG*) for the formation of a critical nucleus of Thiourea single crystals grown from solution using classical homogenous nucleation theory. These will be helpful to study the growth mechanism of Thiourea crystal for computer simulation technique based on the diffusion model.
2.0 ExperimentalThiourea is an organic material, Its molecular weight is 76.12 g/mol. An analytical graded pure Thiourea powder supplied by RanKem is used for crystallization Thiourea powder was dissolved in to double distilled water, the solution is stirred for an hour using magnetic stirrer to ensure homogeneous temperature and concentration through out the volume of the solution. After attaining the saturation, the quilibrium concentration of the solute was analyzed gravimetrically. The experiment was carried out for various temperature ranges as 30, 35, 40, 45 and 50 °C . The nucleation of Thiourea in water occurs is determined by the supersaturation of the Thiourea molecules in water. Thus, higher supersaturation produces more stable aggregates (due to higher probability of collision of diffusing molecules) and therefore increases the formation of stable nuclei. The time period that elapses between the attainment of supersaturation and appearance of a visible speck is defined as induction period
(τ). In the present work, the induction period of Thiourea in water has been measured by the visual observation method. [7.]
2.1 Solubility of Thiourea.
It is necessary to study the solubility of a material to study its growth kinetics The solubility study for Thiourea in water was carried for the temperature range 30-55°C. To determine equilibrium concentration a solution of Thiourea were prepared using double distilled water as the solvent. The beaker containing the solution was covered at the top to prevent evaporation. The solution was continuously stirred using a magnetic stirrer to maintained the homogeneity of temperature and concentration throughout the volume of the solution. After attaining the saturation, the content of the solution was analyzed gravimetrically and this process was repeated for every temperature. The experimentally measured solubility of Thiourea in water for different temperature is shown in figure - 1. The solubility curve shows that Thiourea has a positive solubility- temperature gradient and solution.
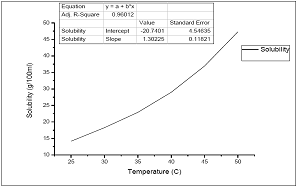
Figure 1: Solubility of Thiourea in water
2.2 The metastable zone width and induction period of Thiourea
The metastable zone width of Thiourea was measured by polythermal method [8]. In this method, the equilibrium-saturated solution is cooled from the overheated temperature until the first visible crystal is observed.Since the time taken for the formation of the first visible crystal after the attainment of critical nucleus is very small, the first crystal observed may be taken as the critical nucleus. The metastable zone width depends not only on the temperature but also on the type of the crystal and its physico-chemical properties . The metastable zone width of Thiourea as a function of temperature is shown
In figure 2. Induction period was measurement by isothermal method [9]. The saturated solution was cooled to the desired temperature and by maintaining at that temperature the time taken for the formation of the first crystal was noted. Repeated trials were performed to ascertain the correctness of the observed results.
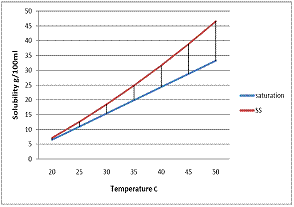
Figure 2 Metastable Zone width of Thiourea.
For crystal growth purpose the super saturated solution is poured in to neat and clean petri dish, which is kept in undisturbed condition for nearly 25 to 30 days. After 3 to 4 days good hexagonal and tetragonal shaped crystals were seen. The average size of crystals was varied in size from 40mmX28mmX1mm to 1.5mmX1.5mmX0.5mm. The transparency of crystals are also varied from opaque to fairly transparent. The grown crystals are shown in Figure 3.


Figure 3: Grown of Thiourea in water
2.3 Nucleation parameters
Interfacial tension in the present investigation has been calculated on the basis of the classical theory of homogeneous formation of spherical nuclei [10]. In practical application the dependence of induction time on interfacial energy can be describe as![]()
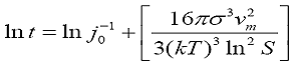
Where is the interfacial energy, Vm is molar volume, S is the supersaturation ratio. This equation clearly shows a linear relation of induction time (ln t). The plot of induction time against (1/ln2S) is shown in Figure 5. The plot is then linearly smooth fitted, standard deviation is comes out 0.316 and its slope is noted. According to above linear equation the slope of the equation can be written as
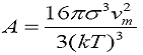
This result of the slope can be used to determine the solid liquid interfacial energy
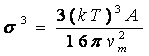
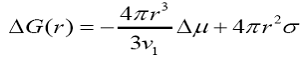
where ΔGv = Δμ /v1is the energy per unit volume, σis the interfacial tension and ris the radius of the nucleus. At critical state, the free energy formation obeys the condition d (ΔG)/dr =0. Hence, the radius of the critical nucleus is expressed as
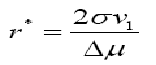
In terms of supersaturation it can be written as:
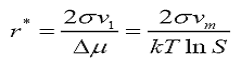
Where S is the supersaturation ratio (c/c0), c is the solution concentration and c0 is the equilibrium saturation concentration. The critical free energy barrier
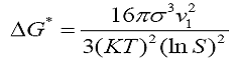
The number of molecules in the critical nucleus is expressed as
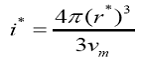
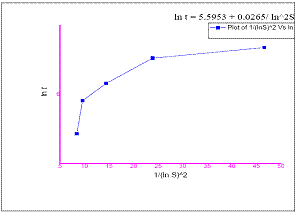
Figure 4: Plot of ln t Vs (1 / ( ln S )^2
The nucleation rate J has been calculated using the equation
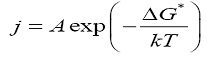
Interfacial tension has been calculated at various constant temperatures from the graph drawn between lnτ and 1/(ln S)2 figure. 4. To
study the effect of supersaturation, induction period experiments were performed for various supersaturation at a particular temperature (40°C). It was observed that the induction period decreases ith increase in supersaturation as shown in figure 4.
A large size Thiourea an organic crystal can be grown under different light frequencies using slow evaporation technique. Its optical property ( i.e. transparency )and size can be improved using different frequencies of light. Result can be tabulated as follow This technique has wide scope to develop new generation of semi organic crystals for NLO applications.
Table – 1 : Effect of frequencies on Growth
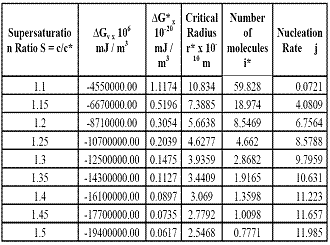
Good quality single crystals of Thiourea were grown by slow evaporation technique. The meta stable zone width and induction period values for Thiourea have been determined from which the interfacial energy values have been calculated as 2.4653 mJ using the experimentally determined induction period values. Nucleation kinetics and fundamental growth parameters have also been investigated. The evaluated nucleation parameters are found to be feasible for the growth of bulk Thiourea crystals.
ACKNOWLEDGMENT
The author is grateful for the support and motivation received from Dr. V.K. Wadhawan. The author feels much indebted to Dr. Arup Banerjee and Dr. V.S. Tiwari for valuable discussions and a critical reading of this manuscript. And also grateful higher education department of Gujarat government, Gandhinagar for inspiration
REFERENCES :
***************************************************
D.G.Adroja
Department of Physics, Jayendrapuri College of Science
Bharuch
Department of Physics, Veer Narmad South Gujarat University
Surat



Home | Archive | Advisory Committee | Contact us